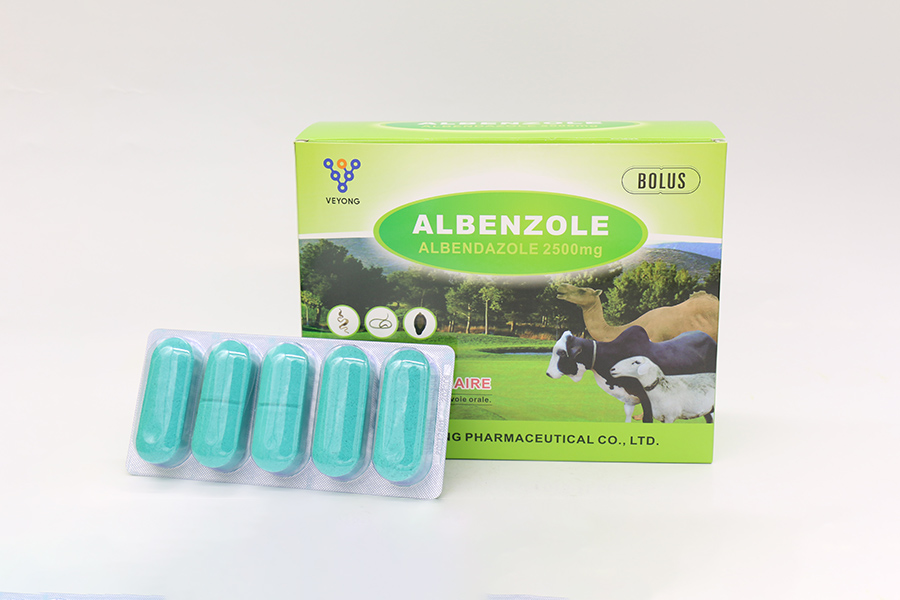
2500mg Albendazole Bolus
Indication
This product is a highly effective and broad-spectrum anthelmintic, mainly used to treat gastrointestinal infections caused by loop nematodes, liver flukes, parasitic lungworms and bisexual animals. Albendazole also has the effect of killing eggs. In the benzimidazole drugs, the anthelmintic spectrum is wider and the insecticidal effect is the strongest. It has high activity against nematodes, schistosomiasis and tapeworms, and can significantly inhibit the development and growth of eggs. Albendazole has a good effect on the treatment of parasites, but it is not effective on insects.
This product can also obviously repel all kinds of parasitic animals, such as schistosomiasis, nematodes, cysticercosis, etc. It can be widely used in livestock. Albendazole can also be used to treat trichinosis, with an effective rate of up to 100%, which is higher than mebendazole.
Cattle can absorb 50% of the administered dose from the gastrointestinal tract. 47% of the oral drug metabolites can be recovered from urine within 9 days. After oral administration of sheep, because it is quickly metabolized to albendazole sulfoxide (the main active metabolite of anthelmintic effect), the original drug cannot be detected in the blood or can only be detected for a short time.
Dosage And Administration
Cattle,Sheep, Camel
Dose: 20mg per kg body weight.
Anthelmintic spectrum:
Fasciola hepatica, heads and segments of Moniezia spp, adults and fourth stage larvae of Ostertagia, Haemonchus, Trichostryongylus, Nematodirus, Cooeria, Dictyocaulus, adult, Bunostomum, Oesophagostomum.
Adverse Effects
Teratogenic effects have been shown for albendazole, cambendazole, oxfendazole and parbendazole, If these drugs are to be used in early pregnancy ,it must be for good reason and at the lowest recommended dosgae.
Withdrawal Time
27days before slaughter.
Not for use in dairy cattle of breeding age, or in any cattle during the first 45 days of pregnancy (or first 45 days after removing bull)
Presentation
5 bolus in a blister, 10 blisters in a box.
Storage
Store in the place below 30℃.
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.







.png)
.png)
.png)
.png)










