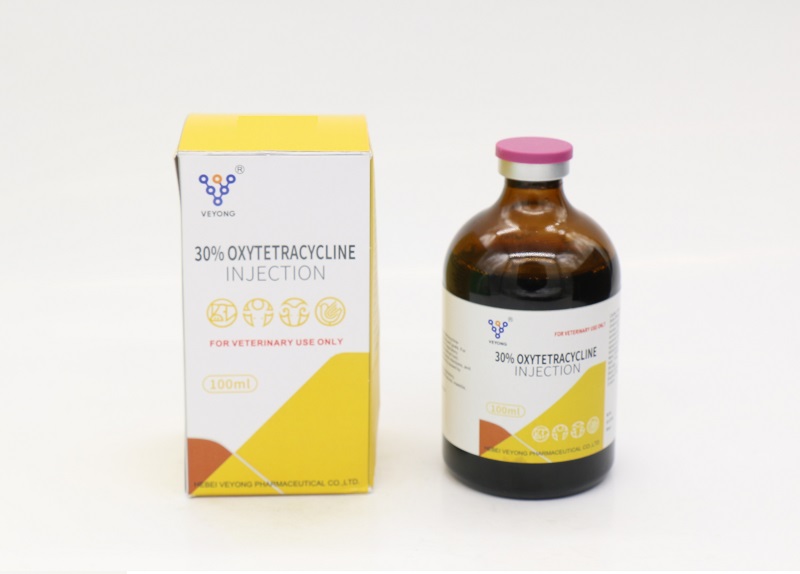
30% Oxytetracycline Injection
Composition
Each 1 ml contains :
Oxytetracycline base.............................300mg
Indications
Oxytetracycline 30% injection is intended for use in treatment for the following diseases when due to Oxytetracycline-susceptible organisms: Beef cattle, non-lactating dairy cattle, calves, including pre-ruminating ( veal ) calves.
Oxytetracycline 30% injection is indicated in the treatment of pneumonia and shipping fever complex associated with steurella spp. , and Histophilus spp.
Oxytetracycline 30% injection is indicated for the treatment of infectious bovine keratoconjunctivitis ( pink eye ) caused by Moraxella bovis , foot - rot and diphtheria caused by Fusobacterium necrophorum: bacteral enteritis ( scours ) caused by Escherichia coli ; wooden tongue caused by Actinobacillus lignieresii ; leptospirosis caused by Leptospira pomona: and wound infections and acute metritis caused by strains of staphylococcal and streptococcal organisms sensitive to Oxytetracycline .
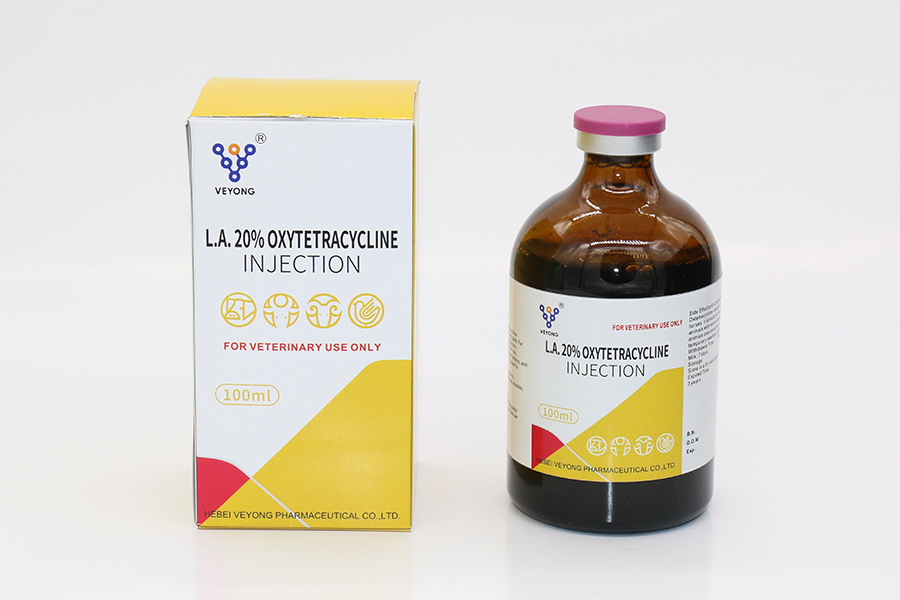
Dosage and administration
By deep Intramuscular injection or Subcutaneous injection
Cattle, Sheep :
Standard Dose : 20mg / kg ( 1 ml / 15 kg )
High Dose : 30mg / kg ( 1 ml / 10kg )
Maximum recommended dosage at one site :
Cattle 15 ml; Sheep 5 ml
Withdrawal period
Meat : Animals must not be slaughtered for human consumption during treatment .
20mg / kg dose : Cattle and sheep after 2 8 days from the last treatment .
30mg / kg dose : Cattle after 3 5 days from the last treatment .
Sheep after 28 days from the last treatment .
Milk : 10days .
Precautions
Exceeding the highest recommended level of drug per kilogram of body weight per day, administering more than the recommended number of treatments, and/ or exceeding 1mL intramuscularly or subcutaneously per injection site in adult beef cattle and non-lactating dairy cattle , may result in antibiotic residues beyond the withdrawal time .
Consult with your veterinarian prior to administering this product in order to determine the proper treatment required in the event of an adverse reaction. At the first sign of any adverse reaction , discontinue use of the product and seek the advice of your veterinarian. Some of the reactions may be attributable either to anaphylaxis (an allergic reaction) or to cardiovascular collapse of unknown cause .
Shortly after injection treated animals may have transient hemoglobinuria resulting in darkened urine .
Storage
Protect from direct sunlight and store below 30 ℃.
Hebei Veyong pharmaceutical Co., Ltd, was established in 2002, located in Shijiazhuang City, Hebei Province, China, next to the Capital Beijing. She is a large GMP-certified veterinary drug enterprise, with R&D, production and sales of veterinary APIs, preparations, premixed feeds and feed additives. As Provincial Technical Center, Veyong has established an innovated R&D system for new veterinary drug, and is the nationally known technological innovation based veterinary enterprise, there are 65 technical professionals. Veyong has two production bases: Shijiazhuang and Ordos, of which the Shijiazhuang base covers an area of 78,706 m2, with 13 API products including Ivermectin, Eprinomectin, Tiamulin Fumarate, Oxytetracycline hydrochloride ects, and 11 preparation production lines including injection, oral solution, powder, premix, bolus, pesticides and disinfectant, ects. Veyong provides APIs, more than 100 own- label preparations, and OEM & ODM service.
Veyong attaches great importance to the management of EHS(Environment, Health& Safety) system, and obtained the ISO14001 and OHSAS18001 certificates. Veyong has been listed in the strategic emerging industrial enterprises in Hebei Province and can ensure the continuous supply of products.

Veyong established the complete quality management system, obtained the ISO9001 certificate, China GMP certificate, Australia APVMA GMP certificate, Ethiopia GMP certificate, Ivermectin CEP certificate, and passed US FDA inspection. Veyong has professional team of registeration, sales and technical service, our company has gained reliance and support from numerous customers by excellent product quality, high-quality pre-sales and after-sales service, serious and scientific management. Veyong has made long term cooperation with many internationally known animal pharmaceutical enterprises with products exported to the Europe, South America, Middle East, Africa, Asia, etc. more than 60 countries and regions.



.png)
.png)
.png)
.png)